David R., Arvanitidis C., Çinar M.E., Dubar J., Dubois S., Erga Z., Guillemain D., Sartoretto S., Thierry de Ville d’Avray L., Zuberer F., Chenuil A., Féral J.-P., (2014), with contributors : Açik Çinar S., Andral B., Aurelle D., Aysel V., Bakir K., Bellan G., Bellan-Santini D., Bouchoucha M., Celik C., Chatzigeorgiou G., Chatzinikolaou E., Chenesseau S., Dağli E., Dailianis T., Dimitriadis C., D’Iribarne C., Doğan A., Dounas C., Egea E., Elguerrabi W., Emery E., Evcen A., Faulwetter S., Gatti G., Gerovasileiou V., Güçver S.M., Issaris Y., Katağan T., Keklikoglou K., Kirkim F., Koçak F., Koutsoubas D., Marschal C., Önen M., Önen S., Öztürk B., Panayiotidis P., Pavloudi C., Pergent G., Pergent-Martini C., Poursanidis D., Ravel C., Reizopoulou S., Rocher C., Ruiton S., Sakher S., Salomidi M., Sarropoulou E., Selva M., Sini M., Sourbes L., Simboura N., Taşkin E., Vacelet J., Valavanis V., Vasileiadou A., Verlaque M. 2014 – Protocols for monitoring of coralligenous habitats of mediterannean (Coralligenous based Indicators to Evaluate and Monitor the “good ecological status” of the MEDiterranean coastal waters) Protocoles de suivi du coralligène en méditerranée (Coralligenous based Indicators to Evaluate and Monitor the “good ecological status” of the MEDiterranean coastal waters) – PDF
presented
– to the 5th International Symposium on the Monitoring of Mediterranean coastal areas: problems and measurement techniques, Livorno (Italy) 17-18-19/06/2014, published in 2014
– and to the RAC/SPA 2nd Mediterranean Symposium on the Conservation of coralligenous and other calcareous bio-concretions, Portorož (SI), 29-30/10/2014, published in 2015
David (R.), Dubois (S.), Erga (Z.), Guillemain (D.), Thierry de Ville d’Avray (L.), Arvanitidis (C.), Çinar (M.), Sartoretto (S.), Zuberer (F.), Chenuil (A.), Féral (J.-P.) 2014 CIGESMED’s protocol and network (Coralligenous based indicators to evaluate and monitor the “Good Environmental Status” of Mediterranean coastal waters). Proc. 5th Intn. Symp. Monitoring of Mediterranean coastal areas: problems and measurement techniques, Livorno (Italy) 17-18-19 June 2014, F. Benincasa (Ed.), pp. 828-843, CNR-IBIMET: Florence (IT), ISBN 978-88-95597-19-5, DOI: 10.5281/zenodo.5759613
David (R.), Arvanitidis (C.), Çinar (M.E.), Sartoretto (S.), Dogan (A.), Dubois (S.), Erga (Z.), Guillemain (D.), Thierry de Ville d’avray (L.), Zuberer (F.), Chenuil (A., Féral (J.-P.) 2015CIGESMED. Protocols: how to implement a multidisciplinary approach on a large scale for coralligenous habitats surveys. RAC/SPA 2nd Mediterranean Symp. on the Conservation of coralligenous and other calcareous bio-concretions, Portorož (SI), 29-30/10/2014, pp. 66-71, DOI: 10.13140/2.1.1895.0086
Reminders on the concepts of coralligenous facies
[§ 1] Coralligenous habitats, considered as underwater landscapes or as ecological puzzles (UNEP – MAP – RAC/SPA, 2009), have a very complex structure that allow the development of several community types (Laborel, 1961; Laubier, 1966; Hong, 1982; Laborel, 1987 ; Ballesteros, 2006).
[§ 2] The description of these facies according to the appearance of different biocenosis is influenced by the predominance of one or more notable species (gorgonians, sponges, erect bryozoans…) favoured by the local preponderance of certain physico-chemical and geomorphological factors (current, fine particules input, light and depth, rugosity, slope and orientation).
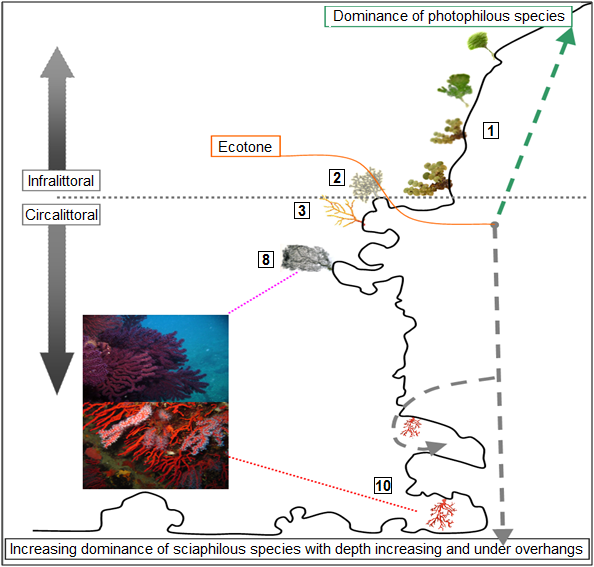
[§ 3] The description of these facies based on their bionomic appearance only remains difficult, and their distribution is very related to surrounding micro-conditions (Virgilio et al., 2006),mainly natural light and currents, that influence the occurrence and the location of various ecotons? and enclaves.
[§ 4] Establish a precise typology and standardize the description of coralligenous facies encountered in the different regions studied under CIGESMED is essential. This approach will be intended through the creation of ontology for this type of habitat?.
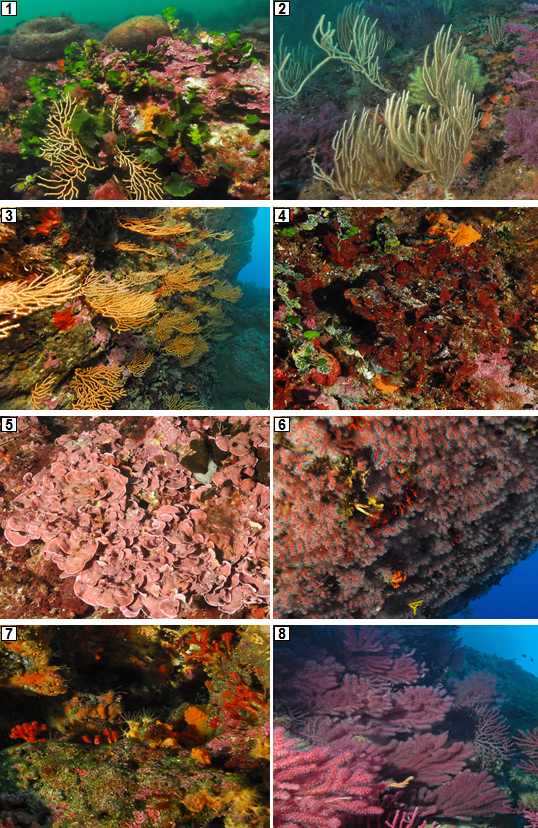
Some facies recognized as coralligenous habitats observed in Marseilles region, are represented in Figure 1.
[§ 5] If we focus on sciaphilic? communities with basal concretions made of coralline algae (Lithophyllum and Mesophyllum) from the infralittoral? and circalittoral? zones, a multitude of remarkable facies are cited in literature:
1) “Pre-coralligenous” facies characterized by the presence of hemiphotophilic algae such as: Udotea petiolata ((Turra) Borgesen, 1926) and Halimeda tuna ((J.Ellis & Solander) J.V.Lamouroux, 1816). This facies has been described by several authors (e.g. Pérès & Picard, 1964; Gili & Ros, 1985; Ros et al., 1985; Gori et al., 2011).
2) Facies characterized by Eunicella singularis (Esper, 1791).
3) Facies characterized by Eunicella cavolini (Koch, 1887).
4) Facies characterized by Peyssonnelia squamaria ((S.G.Gmelin) Decaisne, 1842).
5) Facies characterized by Pseudolithophyllum cabiochiae rims.
6) Facies characterized by Lithophyllum incrustans (R.A.Philippi, 1837).
7) Facies characterized by Mesophyllum alternans ((Foslie) Cabioch & Mendoza 1998).
8) Facies characterized by Paramuricea clavata (Risso, 1826).
9) Facies characterized by Myriapora truncata (Pallas, 1766).
10) Facies characterized by Corallium rubrum (Linnaeus, 1758).
[§ 6] The SPN (service of natural heritage of the French national Museum of natural history) listed Mediterranean biocenosis in a more completed way, in which we can find these facies of coralligenous environment:
IV.3.1. Coralligenous biocenosis (C)
IV.3.1.a. Association? of Cystoseira zosteroides (Syn. C. opuntioides)
IV.3.1.b. Association of Sargassum spp.
IV.3.1.c. Association of Laminaria rodriguezii on rocks
IV.3.1.d. Association of Flabellia petiolata and Peyssonnelia squamaria
IV.3.1.e. Association of Halymenia floresia and Halarachnion ligulatum
IV.3.1.f. Association of Rodriguezella spp.
IV.3.1.g. Association of Lithophyllum spp. and Mesophyllum spp.
IV.3.1.h. Facies of Eunicella cavolini
IV.3.1.i. Facies of Eunicella singularis / Eunicella verrucosa
IV.3.1.j. Facies of Leptogorgia sarmentosa
IV.3.1.k. Facies of Paramuricea clavata
IV.3.1.l. Facies of Parazoanthus axinellae
IV.3.2. Biocenosis of coralligenous shelf
[§ 7] Within the context of CIGESMED, we will consider mainly the biocenosis present in the studied regions and represented in the most typical and common profiles (if they are not enough common, sampling could be avoided) (IV.3.1.d., IV.3.1.g., IV.3.1.h., IV.3.1.i., IV.3.1.k., IV.3.1.l. in bold in the list above). Moreover, other profiles sheltering remarkable species will be taken in account independently (Corallium rubrum, Leptosammia spp.) See examples in Figure 2.
References:
Athanasiadis, A. (1999). The taxonomic status of Lithophyllum stictaeforme (Rhodophyta, Corallinales) and its generic position in light of phylogenetic considerations. Nordic Journal of Botany, 19(6), 735–746.
Ballesteros, E. (2006). Mediterranean coralligenous assemblages: a synthesis of present knowledge. Oceanography and Marine Biology: An Annual Review, 44, 123–195.
Borja A., Bricker S.B., Dauer D.M., Demetriades N.T., Ferreira J.G., Forbes A.T., Hutchings P., Jia X., Kenchington R., Marques J.C., Zhu C., (2008). Overview of integrative tools and methods in assessing ecological integrity in estuarine and coastal systems worldwide. Marine Pollution Bulletin 56, 1519–1537.
Borja A., Ranasinghe A., Weisberg S., (2009). Assessing ecological integrity in marine waters, using multiple indices and ecosystem components: challenges for the future. Marine Pollution Bulletin 59, 1–4.
Boudouresque C.F. (1971). Méthodes d’étude qualitative et quantitative du benthos (en particulier du phytobenthos). Téthys 3: 79-104.
Boudouresque C.F., 2013. Excursion au Cap-Croisette (Marseille) : le milieu marin. 13ème édition. GIS Posidonie publishers, Marseilles : 1-52.
Bråthen K. A., Hagberg O. (2004) More efficient estimation of plant biomass. J Veg Sci 15: 653–660.
Bråthen, K. A., Ims, R. a., Yoccoz, N. G., Fauchald, P., Tveraa, T., & Hausner, V. H. (2007). Induced Shift in Ecosystem Productivity? Extensive Scale Effects of Abundant Large Herbivores. Ecosystems, 10(5), 773–789. doi:10.1007/s10021-007-9058-3
Broom, J. E. S., Hart, D. R., Farr, T. J., Nelson, W. a, Neill, K. F., Harvey, A. S., & Woelkerling, W. J. (2008). Utility of psbA and nSSU for phylogenetic reconstruction in the Corallinales based on New Zealand taxa. Molecular phylogenetics and evolution, 46(3), 958–973. doi:10.1016/j.ympev.2007.12.016.
Claudet J., Fraschetti S. (2010) Human-driven impacts on marine habitats: a regional meta-analysis in the Mediterranean Sea. Biological Conservation 143(9): 2195–2206. doi: 10.1016/j.biocon.2010.06.004
Feldmann J. (1937). Recherches sur la végétation marine de la Méditerranée: la côte des Albères. Wolf. Rouen. 339 pp.
Gili J.M. & Ros J. (1985). Study and cartography of the benthic communities of Medes islands (NE Spain). P.S.Z.N.I: Marine Ecology, 6(3): 219-238.
Glasby TM (2000) Surface composition and orientation interact to affect subtidal epibiota. J Exp Mar Biol Ecol 248:177–190.
Gori, A., Rossi, S., Linares, C., Berganzo, E., Orejas, C., Dale, M. & Gili, J.M, (2011) Size and spatial structure in deep versus shallow populations of the Mediterranean gorgonian Eunicella singularis (Cap de Creus, Northwestern Mediterranean Sea)” Marine Biology, DOI: 10.1007/s00227-011-1686-7
Harvey, E., Fletcher, D., Shortis, M., and Kendrick, G. 2004. A comparison of underwater visual distance estimates made by scuba divers and a stereo-video system: implications for underwater visual census of reef fish abundance. Marine and Freshwater Research 55, 573-580.
Hong, J. S. (1982). Contribution to the study of populations of the coralligenous concretionary bottom from the Marseille region on the northwestern Mediterranean coast. Bull. Korea Ocean Res. Dev. Inst., 4, 1–2.
Kipson S., Fourt M., Teixidó N., Cebrian E., Casas E., Ballesteros E., Zabala M., Garrabou J. (2011) Rapid Biodiversity Assessment and Monitoring Method for Highly Diverse Benthic Communities: A Case Study of Mediterranean Coralligenous Outcrops. PLoS ONE 6(11): e27103. doi:10.1371/journal.pone.0027103.
Laborel, J. (1961). Le concretionnement algal « coralligène » et son importance géomorphologique en Mediterranée. Recueil Travaux Station Marine d’Endoume, 23, 37-60.
Laborel, J. 1987. Marine biogenic constructions in the Mediterranean. A review. Sci. Rep. Port-Cros Natl. Park, 13 : 97-126.
Laubier, L. (19 ). Le Coralligène des Albères, monographie biocénotique. Annales Institut Océeanographie de Monaco, 43, 139-316.
Michez N., Dirberg G., Bellan-Santini D., Verlaque M., Bellan G., Pergent G., Pergent-Martini C., Labrune C., Francour P., Sartoretto S., (2011). Typologie des biocénoses benthiques de Méditerranée, Liste de référence française et correspondances. Rapport SPN 2011 – 13, MNHN, Paris, 48 pages.
Pelletier D., Garcìa-Charton J.A., Ferrarris J., David G., Thébaud O., Letourneur Y., Claudet J., Amand M., Kulbicki M., Galzin R. (2005). Designing indicators for assessing the effects of marine protected area on coral reef ecosystems : A multidisciplinary standpoint. Aquatic Living Ressources, 18, 15-33.
Pelletier D., Claudet J., Ferraris J., Benedetti-Cecchi L., Garcia-Charton J. A.. (2008). Models and indicators for assessing conservation and fishery-related effects of marine protected areas. Canadian Journal of Fisheries Aquatic Sciences, 65(4): 765-779.